HYDROCEPHALUS AND SHUNTS: WHAT THE NEUROLOGIST SHOULD KNOW
اضغط على الرابط التالي لزيارة المقال
HYDROCEPHALUS AND SHUNTS: WHAT THE NEUROLOGIST SHOULD KNOW.
جارى فى اقرب وقت ترجمة مثل هذه المقالات
ومن لديه القدرة على الترجمة عليه ان يتصل بمدير الموقع على
+2 01003646610
المقال
HYDROCEPHALUS AND SHUNTS: WHAT THE
NEUROLOGIST SHOULD KNOW
- Correspondence to: Mr Ian K Pople, Department of Neurosurgery, Frenchay Hospital, Frenchay Park Road, Bristol BS16 1LE, UK: ikpople@hotmail.com
The second most common reason for being sued for negligence in neurosurgery is a problem related to hydrocephalus management (the first being spinal surgery!). However, the good news is that the overall standard of care for patients with hydrocephalus appears to have greatly improved over the last 10 years with the advent of better facilities for investigation, new approaches to treatment, and a greater awareness of the need for adequate follow up. In the possible absence of a local neurosurgeon with an interest in hydrocephalus, a neurologist who is faced with the ongoing care of a patient with hydrocephalus should ideally have a clear idea of what exactly constitutes appropriate follow up and which clinical and radiological warning signals of shunt problems to look out for.
DEFINITIONS
Hydrocephalus is an excessive accumulation of cerebrospinal fluid (CSF) within the head caused by a disturbance of formation, flow or absorption.
“Hydrocephalus ex vaccuo” is a misnomer. It refers to asymptomatic ventricular enlargement caused by generalised loss of cerebral tissue, from severe head injury, infarction or cerebral hypoxia.
“Normal pressure hydrocephalus” is also a misnomer. It describes a condition in older adults of low grade hydrocephalus with intermittently raised intracranial pressure (ICP) (usually at night) causing the classic Adam’s triad of symptoms—gait apraxia, incontinence, dementia.
BASIC HYDROCEPHALUS PATHOPHYSIOLOGY IN ADULTS
The normal CSF production rate in an adult is 0.35 ml/min (20 ml/hour or 500 ml/24 hours). The capacity of normal lateral and third ventricles is approximately 20 ml, whereas the total CSF volume in an adult is 120–150 ml. Hence, in normal circumstances CSF is recycled over three times each day.
ICP rises if production of CSF exceeds absorption, but CSF production will fall as ICP rises to high levels, and compensation (stabilisation of hydrocephalus at a new steady state) may occur through transventricular absorption of CSF. Dural absorption may also be important through nerve root sleeves and unrepaired meningocoeles. Of vital significance is the fact that compensated hydrocephalus (for example, in patients with a longstanding non-functional shunt) is not necessarily permanent because of the sometimes precarious nature of the balance between production and absorption of CSF. Clinicians involved in the care of patients with so called stable hydrocephalus should always be alert to the possibility of insidious subclinical progression or late decompensation of this condition, that may occur spontaneously or after a minor head injury.
As hydrocephalus develops the temporal and frontal horns dilate first, often asymmetrically. There is then elevation of the corpus callosum and stretching of the white matter tracts followed by thinning of the convexity grey matter of the brain (fig 1).
CAUSES OF HYDROCEPHALUS
The causes of hydrocephalus are listed in table 1.
Causes of hydrocephalus
CLINICAL FEATURES
The clinical features of hydrocephalus are notoriously variable, depending on the rapidity of onset of the condition (see box). The most rapid deteriorations are seen in young adults with colloid cysts of the third ventricle where the acute rise in ICP caused by the ball valve plugging of the third ventricle can lead to sudden death. The least rapid presentations occur in older patients with soft compliant brains where the only clue to the presence of progressive hydrocephalus may be a subtle slowing of gait or mentation. This slow presentation also characteristically occurs after severe head injury or subarachnoid haemorrhage.
INVESTIGATIONS
Computerised tomography
A computerised tomographic (CT) scan should be undertaken to assess the overall size of the ventricles, and to determine if periventricular oedema or “lucency” is present. A CT scan is also useful to assess the size of the fourth ventricle—if large, this suggests a communicating hydrocephalus, whereas a relatively small fourth ventricle implies obstructive hydrocephalus that might be best treated by endoscopic third ventriculostomy rather than a ventriculo-peritoneal shunt.
Magnetic resonance imaging
Chiari malformations and cerebellar or periaqueductal tumours, sometimes not visible on CT, can be detected with magnetic resonance imaging (MRI). This imaging technique is also useful for detecting flow voids in the third ventricle and aqueduct. Various radiological features (angle of the frontal horns on coronal view, downward bowing of the floor of the third ventricle, elevation of the corpus callosum) may imply active hydrocephalus, but these must not be heavily relied upon because of their poor sensitivity. Differentiating normal pressure hydrocephalus from cerebral atrophy is still often more of an art than a science.
ICP monitoring/CSF infusion studies
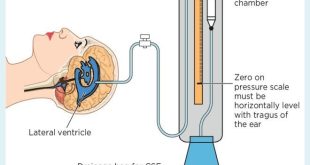
ICP monitoring and CSF infusion studies are now being used more frequently in young patients with mild symptoms and older patients with possible low grade hydrocephalus. ICP monitoring may reveal “B waves” either at night time alone or throughout the day and night. An ICP above 15 mm Hg at frequent intervals during the night or day while asleep or resting is abnormal, and patients with functioning shunts should normally have an ICP below or near to zero while 45° head up in bed.
Clinical features of hydrocephalus
- Young adults:
-
Symptoms—headache, vomiting, failing vision, drowsiness, “muzziness of the head”, fatigue
-
Signs—papilloedema, enlarged blind spots on visual field analysis or reduced visual acuity, failure of upward gaze, general clumsiness, dyspraxic gait, large head
-
- Older adults/elderly:
-
Symptoms—slowing of mental capacity, unsteady on feet/frequent falls, incontinence, drowsiness, headaches less frequently
-
Signs—gait dyspraxia (slow, hesitant shuffling gait), dementia (reduced mini-mental score), rarely papilloedema
-
Lumbar CSF infusion tests measure CSF outflow resistance, which in simple terms represents the overall compliance of the intracranial and spinal CSF compartment. During this test saline or artificial CSF is constantly infused via a lumbar puncture needle or catheter, and the subsequent gradient of rise in the ICP with time is recorded. A low outflow resistance corresponds to high cerebral compliance and vice versa. Normal values are 5–10 mm Hg/ml/minute and a value > 18 mm Hg/ml/minute appears to be the approximate cut off point for diagnosing active hydrocephalus in the elderly. Other compliance monitors have recently been developed that are placed as bolts through small twist drill holes in the skull. These tests can be used to guide treatment of patients with newly diagnosed ventricular enlargement; they can also be useful in patients with possible blockage of their shunts or delayed occlusion of their third ventriculostomy site.
Transcranial Doppler
Transcranial Doppler involves the non-invasive measurement of the middle cerebral artery flow velocities and pulsatility index. The latter index appears to correlate fairly well with ventricular distension and cerebrovascular impedance. The quality of information obtained is very much operator dependent, but can be useful for monitoring patients in an outpatient setting.
Tympanic membrane displacement
Measurement of tympanic membrane displacement is an indirect non-invasive technique for assessing ICP. It is based on a direct communication of pressure waves from the intracranial space to the middle ear via the endolymphatic system of the inner ear. There must be a clear ear canal, intact tympanic membrane, and skilled, experienced operators to give dependable results. In order to diagnose a blocked shunt the patient should preferably already have a baseline reading while well with an obviously functioning shunt.
CSF sample
In post-subarachnoid and post-meningitic hydrocephalus, CSF samples are useful for cell counts, protein concentration, and to exclude residual infection. A protein concentration greater than 4 g/l will clog up most ventriculo-peritoneal shunt valves.
Psychometric analysis
Although it is highly unlikely that any patients with possible pressure from hydrocephalus would ever stand a chance of seeing a neuropsychologist during an investigational work up, the right posterior hemisphere has been shown to be most susceptible to functional deterioration from raised ICP. Deterioration in hand–eye coordination and visuospatial skills may precede classic symptoms of shunt blockage.
WHICH TREATMENT OF HYDROCEPHALUS IS BEST: ENDOSCOPIC OR SHUNT?
Since the 1960s the standard treatment of hydrocephalus has been the insertion of a valved ventricular shunt either into the peritoneum or right atrium of the heart. Most neurosurgeons have used ventriculo-peritoneal (VP) shunts mainly because of the potentially life threatening nature of some of the complications of ventriculo-atrial (VA) shunts (multiple pulmonary emboli, shunt nephritis).
Over the past 10 years or so there has been a huge resurgence of interest in ways of avoiding CSF shunts for hydrocephalus by utilising endoscopic techniques, such as third ventriculostomy. During this period smaller endoscopes have been developed that have better optics and brighter light sources, and these changes have undoubtedly enabled neurosurgeons to perform these techniques with a greater degree of confidence and safety for the patient. The attraction of giving patients the chance of escaping the numerous inherent complications of CSF shunts has led to a rapid expansion of enthusiastic neurosurgeons offering a neuroendoscopic alternative for the treatment of hydrocephalus. Techniques such as endoscopic septostomy (making a hole in the septum pellucidum for a trapped ventricle), cyst fenestration or choroid plexus coagulation have been used with reasonable long term success, and colloid cysts and pineal tumours are often ideally treated by skilled neuroendoscopy.
ENDOSCOPIC THIRD VENTRICULOSTOMY
In the past the main indication for third ventriculostomy has been non-communicating (obstructive) hydrocephalus, demonstrated by CT or ventriculography. There would typically be dilatation of the lateral ventricles, ballooning of the third ventricle, and a relatively small fourth ventricle. The aetiology of hydrocephalus was generally congenital aqueduct stenosis, and a history of meningitis would have been regarded a contraindication. However, these traditional criteria are no longer adhered to by an increasing number of neuroendoscopists, who have greatly extended their indications for third ventriculostomy to include patient groups previously regarded as inappropriate.
The technique consists of the creation of a single burrhole in the frontal region followed by ventricular cannulation and insertion of a 3 or 4 mm wide neuroendoscope into the lateral ventricle. The third ventricle is then negotiated via the foramen of Monro and a hole is then made in the floor of the third ventricle between the infundibulum of the pituitary gland and the mammillary bodies (fig 2). This creates a CSF fistula between the third ventricle and the subarachnoid space in front of the brainstem. The hole is made with a small electrode and enlarged with a balloon dilator, such that a very exciting close up view of the basilar artery is unveiled.
View of foramen of Monro via a neuroendoscope in the lateral ventricle.
The cause of hydrocephalus undoubtably influences the chance of long term success. There is clearly a better success rate in patients with aqueduct stenosis, spina bifida, and tectal, pineal, and posterior fossa tumours. The risk of failure appears to increase with a past or recent history of intracranial infection, presumably as a result of obliteration of cerebrospinal fluid pathways. Long term results have been questioned as follow up is often not more than five years in the literature, but most studies quote an overall success rate between 65–75%.
The overall complication rates reported in published series varies widely from 4–30%, but the overall rate of serious complications is 9.4% and this includes an average 3% infection rate, 2.3% haemorrhage rate, and 1.3% risk of a permanent neurological deficit. Fortunately the rate of life threatening complications appears to be low and postoperative deaths are rare (0.1%, or 1 per 1000 third ventriculostomies).
CEREBROSPINAL FLUID SHUNTS
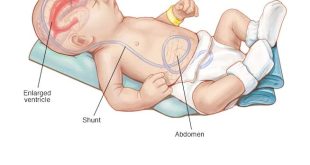
Most shunting systems drain according to the differential pressure gradient between the ventricle and the tip of the distal catheter. These valves have been shown to be effective in the majority of patients and a typical valve is shown in fig 3. Most neurosurgeons use medium pressure valves, that will drain CSF continuously if the differential pressure is over about 10 mm Hg. The ventricular catheter of a shunt is normally inserted through a burrhole in the right parieto-occipital region and the valve will sit usually behind the right ear. The distal catheter is tunneled subcutaneously down to another incision in the abdomen where it is then placed into the peritoneal cavity. It is not usually helpful for non-neurosurgeons to palpate or flush the shunt valve, as their contours and characteristics are so variable as to make interpretation notoriously inaccurate.
Clinical features of shunt malfunction
-
Headaches
-
Vomiting
-
Drowsiness
-
Papilloedema with or without failing vision
-
Occasionally failure of upward gaze
-
Neck stiffness
-
Thoracic back pain in patients with spina bifida
Investigations of shunt malfunction
-
CT scan (enlargement of ventricles)
-
Plain x ray of shunt system (lateral skull, anteroposterior (AP) chest and AP abdomen)
-
Palpation of shunt reservoir—unreliable
-
Peripheral blood for C reactive protein, white cell count if there has been any recent surgery
-
Shunt reservoir tap
-
ICP monitoring/lumbar infusion test
In selected patients a ventricular access device (otherwise known as an “Ommaya reservoir”) is placed in the right frontal region for ICP monitoring or treatment of infections (fig 4). These cannot be flushed or assessed in any way by palpation, but provide the facility for potentially life saving percutaneous aspiration of CSF in the event of acutely raised ICP. This can be done by any clinician in this situation and simply involves the passing of a butterfly needle through the skin perpendicular to the surface of the skin at the apex of the dome of the reservoir, until a “pop” is felt. Elective sampling of reservoirs or shunts should preferably be carried out by a neurosurgeon, unless the clinician looking after the patient has had previous experience in the technique.
Ventricular access reservoir and catheter for percutaneous sampling of CSF.
Differential pressure valves allow the siphoning effect in the upright position and this may lead to excessive CSF drainage from the ventricles. Some systems now incorporate an anti-siphon device to ameliorate this effect.
“Programmable” or adjustable valves allow the closing pressure to be altered externally using a special magnetic adjusting device. Although this is sometimes extremely useful in selected shunted patients with intractable headaches, it can lead to problems following inadvertent change of pressure—for example, by having an MRI scan or, less obviously, by using headphones and certain cordless phones.
Flow controlled valves, such as the Orbis-Sigma valve, have a more physiological CSF drainage pattern, but they do not appear to be effective in normal pressure hydrocephalus or where brain compliance is poor (so called “brittle ventricles”).
COMPLICATIONS OF SHUNTING
Shunt obstruction
Shunt obstruction may occur proximally in the ventricular catheter as a result of choroid plexus, red cells, tumour cells, or a high protein concentration in the CSF. Blockage of the distal catheter can occur as a result of body growth (if the shunt was placed during childhood), adhesions within the abdominal cavity, especially when associated with a low grade infection, pregnancy, and occasionally constipation.
Urgent help from the on-call neurosurgeon should be sought for all suspected cases of acute shunt malfunction as patients with little remaining compensatory reserve may deteriorate suddenly as a result of a respiratory arrest, seizures, or simple coning. Shunt blockage may cause death and blindness if there is a combination of sudden onset and delay in treatment.
Uncomplicated shunt revisions do not affect long term outcome. However, revision surgery on patients with blocked shunts is occasionally complicated by serious secondary ventricular or intraparenchymal haemorrhage, and any patient who is “not quite right” soon after a shunt revision should have a follow up CT scan immediately. Most young patients with shunts require a revision operation once or twice every 10 years as the shunt tubing degenerates gradually over the years and flakes of silicone break off (causing subcutaneous granulomas), weakening the wall of the tube. Eventually the tube may fracture or obstruct.
Some unlucky patients run into multiple problems with their shunt, usually as a result of “one problem leading to another” (fig 5). There is little to be done in such circumstances, other than commiserate with the patient and approach each hurdle in a positive and objective manner. However, when the situation becomes unduly complicated or intractable it may be prudent for the neurologist or neurosurgeon to seek the opinion of a neurosurgeon with a special interest in hydrocephalus.
Multiple shunt catheters caused by intractable shunt failure—a nightmare scenario for the on-call neurosurgical team.
Infection
Shunt infections are usually caused by the patient’s own skin organisms (most common is Staphylococcus epidermidis), which gain access to shunt tubing during the shunt procedure. Typically this contamination will cause an internal shunt colonisation where the bacteria settle and grow on the internal wall of the shunt catheter and valve, establishing adherent colonies. However, some bacteria set up a ventriculitis without full colonisation of the shunt, and others (for example, Staphylococcus aureus) cause an external shunt infection (deep wound infection).
The most important clinical features of a shunt infection are as follows:
-
general malaise
-
pyrexia
-
headaches, vomiting, neck stiffness
-
abdominal tenderness or distension
-
recurrent lower end shunt obstruction
-
occasionally pain and erythema around the shunt
-
pulmonary hypertension or shunt nephritis in chronic VA shunt infections
-
recent shunt operation
-
90% of VP shunt infections present within three months of a shunt operation
-
raised C reactive protein
-
high peripheral and CSF white cell count
-
culture of organism from CSF.
Patients should be reassessed urgently by the relevant neurosurgical team should any of the above symptoms develop within the first few months after a shunt operation. After six months a VP shunt will not become infected unless intra-abdominal sepsis occurs (for example, appendicitis, diverticulitis or postgastrostomy feeding tube insertion).
The current incidence of shunt infection in most neurosurgical units is about 5–8%, but many units are now achieving better results as a result of preventative measures and protocols. Details, including infection rates, of most shunt operations performed in the UK are now submitted to the UK Shunt Registry that was set up by the Medical Devices Agency a few years ago. It is hoped that information derived by analysing this huge data set will lead to further improvements in the standards of shunt surgery in the future.
Infected shunts will often have to be removed and then replaced after two weeks of antibiotics and temporary drainage. Infections with low grade pathogens can sometimes be treated with intraventricular and intravenous antibiotics alone.
Over-drainage
The perfect shunt valve has yet to be designed and many current models allow over-drainage of CSF due to the siphoning effect. The hydrostatic pressure (25–75 cm CSF) caused by the weight of the column of CSF within the distal catheter leads to fluid being sucked out of the ventricles in the upright position. The valve pressure may be set too low for an individual patient leading to over-drainage; this can be remedied by adjustments of the valve pressure either by revision of the valve or by using a programmable valve. ICP monitoring may be required in those patients presenting with possible low pressure headaches without evidence of subdurals on the CT scan.
Subdural haematoma can occur during the first six months after a shunt insertion and has been shown to be related to the amount of CSF released at operation. Small collections occur in up to 30% of patients after shunt insertion in the elderly, but symptomatic collections requiring surgery affect only 10–15%. The symptoms of a shunt related subdural collection include headaches, confusion, hemiparesis, and drowsiness.
FOLLOW UP AFTER ENDOSCOPIC THIRD VENTRICULOSTOMY
There is a clear need for continued follow up of patients after apparently initially successful third ventriculostomy, as late failure occurs in up to 40% and sudden death has been recently reported as little as two years following apparently successful third ventriculostomy.
Most patients are followed up primarily by monitoring their clinical symptoms, optic fundi, and visual acuity. Midline sagittal T2 weighted MRI sequences combined with cine phase contrast MRI flow measurements provide a reliable tool for ascertaining the patency of the stoma during follow up evaluation. The important sign is flow voids through the ostomy; change in ventricular size is less important after third ventriculostomy. However, a 33% reduction in the size of the third ventricle on follow up CT scans occurs in patients whose symptoms have been successfully controlled. Lateral ventricles will only decrease in size on average by 16%.
ICP measurements from reservoirs may be useful in selected younger patients, as the value of ICP monitoring in shunted children by means of a reservoir or by parenchymal devices has been previously well documented. Reservoirs with an integral telesensor to measure ICP non-invasively may prove useful after third ventriculostomy where the preoperative pressures have been substantially raised. Other workers have advocated the use of CSF infusion studies to monitor cerebral compliance, or transcranial Doppler indices before and after operation as an indirect measure of compliance.
HOW SHOULD WE FOLLOW UP SHUNTED PATIENTS?
Arrangements for follow up of adult patients with shunts have been extremely variable, with some patients receiving careful regular annual outpatient assessments and others being completely discharged and left to contact their general practitioners in the event of symptoms. A number of patients have gone blind or died as a result of undetected chronic shunt malfunction, and efforts by the Association of Spina Bifida and Hydrocephalus have led to improvements in standards of follow up care. The principle components of good long term care of patients with shunted hydrocephalus are outlined below.
All patients should have a baseline CT brain scan 6–12 months after their initial shunt insertion, while they are well. They should preferably retain a copy of this baseline scan if they travel far from their base neurosurgical unit, and it is our experience that most patients are willing to pay for this copy. Although it is not always possible to detect shunt blockage on a CT scan as a result of a patient having non-compliant ventricles, this is the exception rather than the rule.
All patients and carers should be given clear instructions (preferably written) as to what symptoms to look out for and when to contact their doctor. Some documentation of exactly which type of valve has been implanted should be given to the patient. Those with programmable/adjustable valves should always have their shunt re-programmed, or at least checked by their neurosurgeon after any MRI scan has been carried out. They should be made aware of the possible problems of inadvertent valve pressure change from extraneous magnetic sources.
All younger (under 60 years) patients should have an annual visual acuity check by an optician or an ophthalmologist if there have been particular concerns about vision.
All younger patients with a shunt should probably be encouraged to seek a neurosurgical check up at least every three years, ideally at a dedicated hydrocephalus follow up clinic.
SUMMARY
Modern trends in surgical treatment of hydrocephalus are moving towards the greater use of minimally invasive endoscopic procedures and away from routine shunting wherever feasible.
Patients with isolated hydrocephalus should have a normal life expectancy, as long as prompt detection and treatment of complications is provided through maintaining appropriate arrangements for long term follow up.
KEY REFERENCES
-
Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with `normal’ cerebrospinal fluid pressure N Engl J Med 1965;273:117–26. ▸ A landmark paper in the clinical assessment of hydrocephalus in the older age group.
-
Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery2001;49:1166–86. ▸ A general overview of diagnostic criteria and treatment of older patients with hydrocephalus.
-
Boon AJ, Tans JT, Delwel EJ, et al. Dutch normal-pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. J Neurosurg1997;87:687–93. ▸ An evaluation of the use of lumbar infusion tests in the diagnosis of active hydrocephalus in older adults. A widely quoted paper on the assessment of normal pressure hydrocephalus.
-
Pople IK, Edwards RE, Aquilina C. Endoscopic management of hydrocephalus treatment. Neurosurg Clin N Am2001;12:719–35. ▸ An overview of current endoscopic techniques in hydrocephalus treatment.
-
Drake JM, Kestle JR, Tuli S. CSF shunts 50 years on – past, present and future. Childs Nervous System 2000;16:800–4. ▸ A useful overview of ventricular shunts for hydrocephalus.